Medical device integration allows the data generated from the devices to flow seamlessly across the information system of a hospital or clinic. It helps medical organizations use and manage the data better and streamlines all the clinical and administrative activities. When doctors can access the latest data on patients in real-time, they can make better decisions regarding the diagnoses and treatments.
Integrating medical device systems facilitates interoperability between healthcare applications, the EHR software, and medical devices. Let’s talk about the process of medical device integration in further detail.
Steps Involved in Medical Device Integration
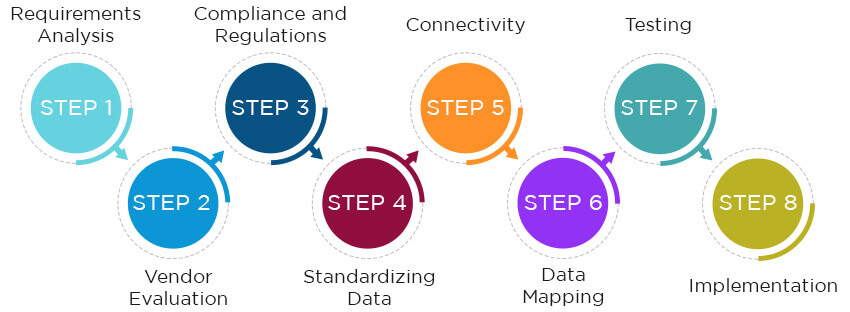
The primary goal of medical integration services is to enable the data from medical devices to flow across the hospital’s health information system and be usable for providers and other staff. The steps for a successful medical device integration process are:
1. Requirements Analysis: Carry out a thorough assessment of the requirements of the healthcare facility. It is important to determine what medical devices need to be integrated with which type of system software.
2. Vendor Evaluation: It is important for the healthcare organization to conduct a thorough research before choosing a vendor for the devices and integration solutions. Consider factors like device compatibility, reputation of the vendors, cost, and scalability.
3. Compliance and Regulations: Organizations looking for medical device integration services must ensure that the devices and the integration process comply with regulations like HIPAA (Health Insurance Portability and Accountability Act) and the rules stipulated by the FDA (Food and Drug Administration).
4. Standardizing Data: The data generated by the medical devices might not be compatible with the health information system at the hospital. That is why it needs to be standardized to be shared and used across the organization. Medical integration services usually use protocols like HL7 and FHIR for standardizing data. They define the structure of the data, allowing seamless transfer between devices, health systems, and EMR/EHR systems.
5. Connectivity: Healthcare integration involves connecting two or more devices to existing systems. The connection is established using adapters for reception. Once they are installed, users can select the right entry from a menu and fill out some fields to finalize the configuration.
6. Data Mapping: Once the data has been standardized and the connection between the systems established, the data must be mapped to the right fields. Mapping the data allows the users to define the relationships between the varying data sets from the medical devices and the existing medical records. This allows the information and readings generated from the devices to be stored in the EHR and then shared across the health system.
7. Testing: It is vital to ensure that the data generated from the medical devices can be transferred over to the EHR. In case of private practices, the data from the devices should flow to the health records and also be accessible from the practice management solutions. The testing simulates data transfer from a device to check for functionality.
8. Implementation: This is the final phase of medical data integration. The integration solution is implemented at a hospital, clinic, or even a private practice.
Regulations and Compliances To Know During Medical Device Integration
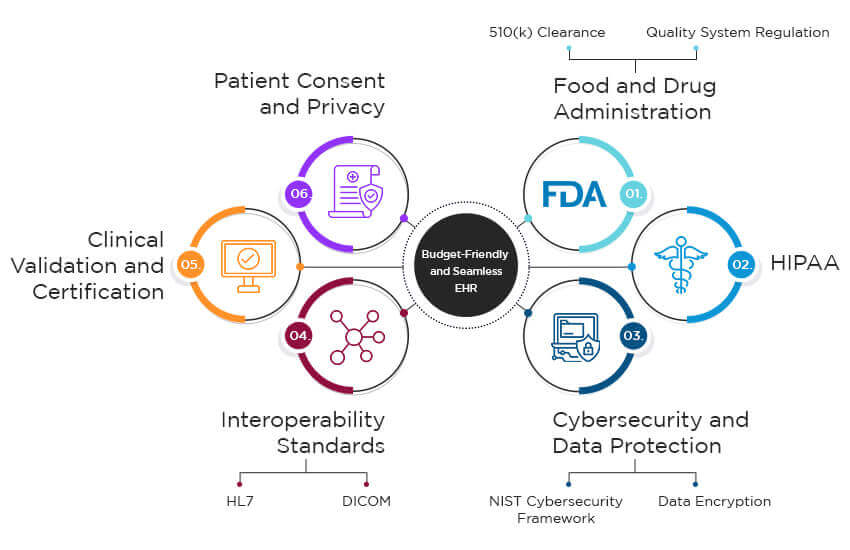
The process of medical device integration software development will enable medical devices to transmit sensitive patient data to health records. So, it is vital to protect the privacy and integrity of this data as it is shared across multiple digital systems. Companies offering medical device integration solutions need to be aware of the following regulations stipulated by the government:
1. Food and Drug Administration (FDA) Regulations:
- 510(k) Clearance or Premarket Approval (PMA): Devices may require prior approvals before integration depending on the level of risk they pose.
- Quality System Regulation (QSR): The QSR or 21 CFR Part 820 is necessary for ensuring that the design, manufacturing, and post-market surveillance of medical devices meet quality standards.
2. HIPAA (Health Insurance Portability and Accountability Act):
This regulation stipulates security measures to protect the privacy and integrity of patients’ data. Healthcare organizations must ensure HIPAA compliance for the medical device integration process.
3. Cybersecurity and Data Protection:
- NIST Cybersecurity Framework: Medical organizations must follow the best practices outlined by the NIST framework to protect sensitive data against unauthorized access and breaches.
- Data Encryption: The data transmitted or stored by integrated healthcare solutions should be encrypted. It will prevent unauthorized access to the data.
4. Interoperability Standards:
- HL7 (Health Level 7): The EHR integration of medical devices or integration with health information systems must follow HL7. This standard ensures seamless data exchange between two or more medical software systems.
- DICOM (Digital Imaging and Communications in Medicine): This standard is required for the sharing of medical images and related data among different systems.
5. Clinical Validation and Certification:
The medical devices must undergo comprehensive validation and verification processes to prove their safety and effectiveness under real-life scenarios.
6. Patient Consent and Privacy:
- Healthcare organizations like hospitals, clinics, and practices must obtain the informed consent of their patients for the collection and use of their data using integrated medical devices.
- Patients must be made aware of how their data will be used and should be educated about privacy policies.
Cutting-Edge Medical Device Integrations To Watch Out For
Integrating medical devices helps providers have the latest data on their patients. The Covid-19 pandemic stimulated the healthcare industry to adopt many forms of telehealth solutions and remote patient monitoring technologies for enabling providers to serve patients without the need for in-person visits. Let’s look at some of the most popular devices to be integrated into health systems:
1. Remote Patient Monitoring Devices
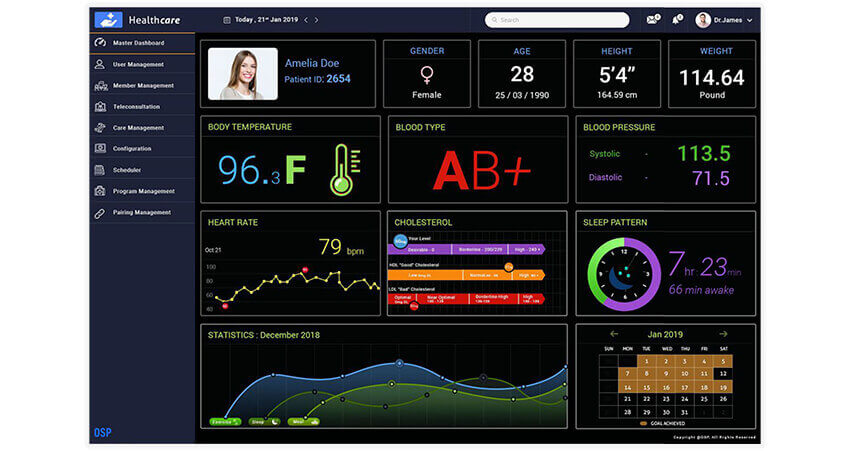
Remote patient monitoring (RPM) devices measure patients’ vital signs outside of conventional clinical settings and send them to providers in real-time. They allows doctors and caregivers to monitor patient health remotely. Common devices used in RPM can include heart rate monitors, blood pressure cuffs, glucose monitors, and others.
Integrating RPM devices with health information systems provides real-time health data for effective remote care. Let’s assume a scenario in which a 70 year old man with diabetes and heart disease begins an RPM program. His care team will provide him with a wireless heart rate monitor, blood pressure cuff, and a glucometer. The man can use these to measure his vital signs and transmit them to his doctor, who then tracks this data to monitor his health remotely. If the vital signs cross a threshold, the provider will receive an alert notification and make an appropriate response.
Transparency Market Research has found that the global market for RPM devices in 2019 was at $0.8 billion. But it is expected to expand at a CAGR of 12.5% from 2020 to 2030. A significant portion of this growth can be expected to be seen in the United States.
2. Augmented Reality and Virtual Reality

Augmented and virtual reality (AR/VR) were conceived as the next big leaps in entertainment. But it was only a matter of time before their applications in healthcare were recognized. These devices include wearable goggles which simulate an immersive, digital world were user can view or interact with things which will feel lifelike.
AR and VR have been recognized to help people suffering from anxiety and phobias. Consider an example where a person with vertigo phobia is considered for treatment using AR technology. He can visit a clinic where the therapist will use the VR goggles to simulate the edge of a roof of a tall building. The man suffering from the vertigo phobia can stand on solid ground knowing full well that he is in a simulation, but feel like he is on the roof of a building. Such a treatment can help to lower the anxiety that comes from being at heights and process the fear better.
3. Fall Detectors

As the name indicates, fall detectors can detect sudden falls and trigger alarms automatically in such cases. These devices are used mainly for senior care in retirement communities and home care. A fall detector mounted at a strategic location at home can monitor the person 24/7 without infringing on his or her privacy. The device can detect a fall and trigger an alarm that would also alert caregivers, family members or emergency dispatch operators who can send an ambulance.
The data generated from a fall detector can be stored in a health record and assessed by providers and caregivers. Repeated patterns of falls and incorrect gait may indicate additional problems. In such cases, providers can prescribe tests for better diagnoses. Fall detectors are especially helpful for seniors and people suffering from neurological or degenerative problems.
4. Maternal and Foetal Monitors
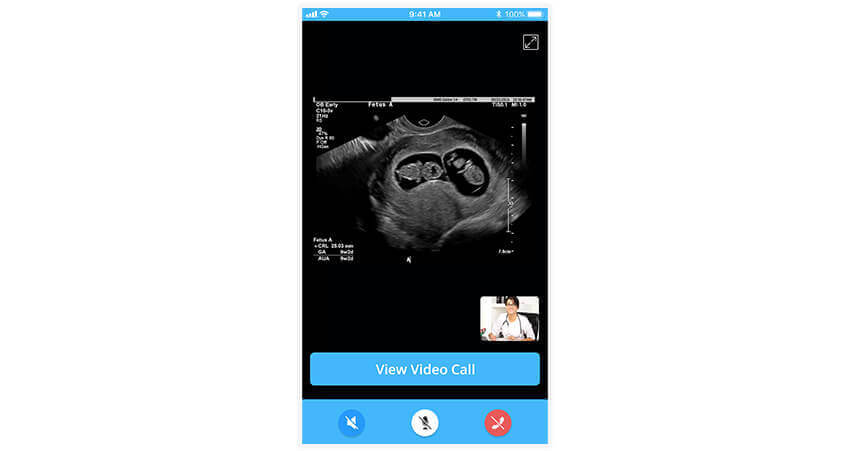
Maternal and foetal monitors (MFM) record vital signs like fetal heart rate, maternal arterial blood oxygen saturation, and contractions of the woman’s uterus, among others. It is essential for prenatal and perinatal care and allows physicians to diagnose any complications accurately. The inclusion of MFM monitors into telehealth applications helps women in rural communities receive prenatal care.
Consider a Medical software integration at a rural clinic in a small town in the United States. Pregnant women can visit such a clinic for their check-ups, where a radiologist can conduct a sonogram and use maternal and foetal monitoring devices for transmission. The data from this visit, including the sonogram itself can be transmitted in real-time to a specialist at a remote location. The specialist can provide an informed diagnoses based on this data to help the rural clinic achieve better outcomes for the women and their foetuses.
5. Remote Sleep Monitors
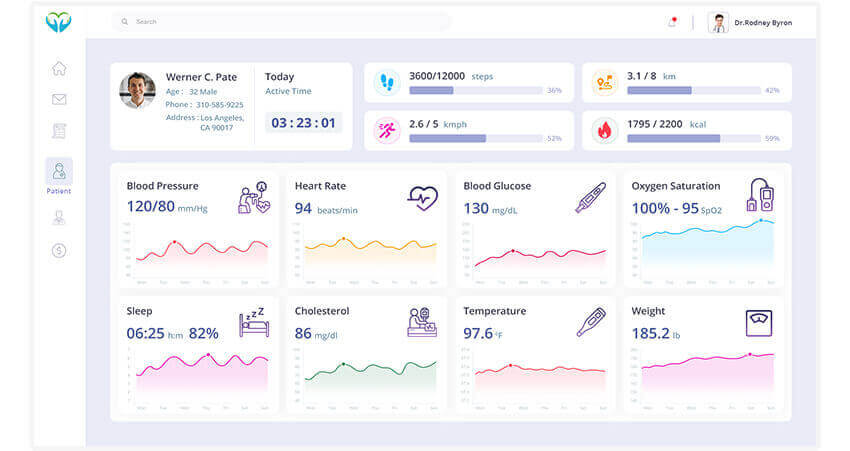
Remote sleep monitors are devices that measure particular vital signs to measure the quality of a person’s sleep. They are highly useful in treating sleep disorders which affect more than 50 million Americans, according the latest finding by the Sleep Foundation. That is almost a fifth of the population of the country.
Medical device integration software for remote sleep monitors can allow the devices to transmit vital signs like respiration, heart rate, temperature, movements, blood pressure, and others. A person suffering from sleeping issues can visit a sleep specialist and obtain remote sleep monitors. The person can wear these monitors or set them up as per instruction beside his bed before sleeping. The devices would monitor the person’s sleep and measure his vital signs. This data can help the specialist diagnose the condition better and prescribe the necessary treatments.
Benefits of Medical Device Integration
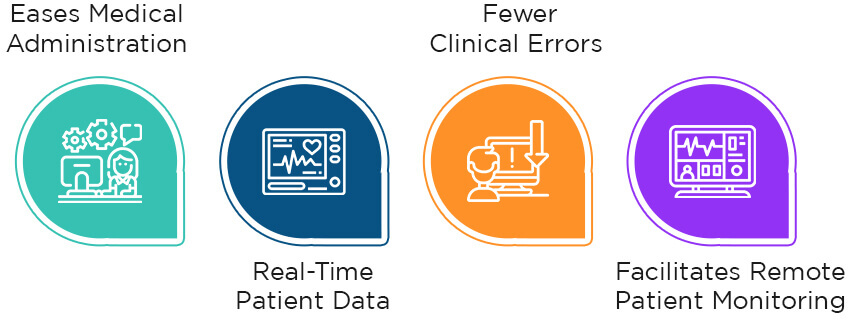
Integrating medical devices with health systems or medical records solutions has many benefits for providers and patients. Let’s explore some of them in detail:
1. Eases Medical Administration
Integrating medical devices collects health data from the patients and stores it directly into patients’ health records. This eliminates the need for the providers or the nursing staff to record this data manually. They can instead focus on other tasks and be more productive across clinical and medical workflows.
2. Real-Time Patient Data
A major benefit of medical device integration is access to patient data in real-time. The patients’ vital signs will be stored in their health records almost as soon as they are measured. Continuous stream of real-time patient information enables providers to track fluctuations or anomalies better and provide more accurate diagnoses.
3. Fewer Clinical Errors
Healthcare software integration of the medical devices with the health information systems eliminates the need for the staff to enter the data manually. The providers can directly view the data on their screens and upload them to the EHR. The lack of need for manual data entry minimizes clinical and clerical errors.
4. Facilitates Remote Patient Monitoring
Integrating medical devices to EHR and EMR systems will enable providers to monitor patient vitals without in-person consultations. People can measure their vitals from the comfort of their homes and transmit the same in real-time to providers. A single doctor can monitor the health of numerous patients remotely and offer medical services that way.
Conclusion
Medical device integration has allowed large hospitals, medium sized clinics, as well as private practices to streamline all their operations and help patients better. As newer innovations and advancements continue to emerge, it will only increase the demand for medical device integration software to accommodate the latest technologies. Companies involved in this ecosystem will have to work together to smoothen the process and comply with evolving regulations.
OSP is a trusted software development company that delivers bespoke solutions as per your business needs. Connect with us to hire the best talents in the industry to build enterprise-grade software.

How can we help?
Fill out the short form below or call us at (888) 846-5382
Looking for software solutions to build your product?
Let's discuss your software solutions for your product in our free development acceleration call!
Get In Touch arrow_forwardDiscuss Your Project Handover with a team of expert Book a free consultation arrow_forward
About Author

Author linkedin
Riken’s work motto is to help healthcare providers use technological advancements to make healthcare easily accessible to all stakeholders, from providers to patients.

Riken Shah linkedin
Riken’s work motto is to help healthcare providers use technological advancements to make healthcare easily accessible to all stakeholders, from providers to patients.