Solutions We Delivered

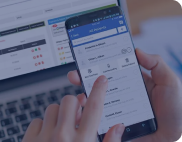
Mental Health PM+RCM Solution
Built a customized solution to improve revenue cycle and practice management workflows in a mental-health center.
reduction in claims losses

Doctors on Demand Platform
Developed a telehealth platform with virtual streaming capabilities to improve care accessibility and patient engagement.
improvement in home care experience


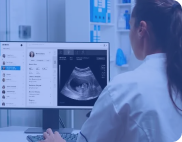
Ultrasound Analysis and Telehealth
Created an AI-powered ultrasound streaming solution with telehealth capabilities to solve real-time remote diagnosis challenges.
improvement in diagnosing abnormalities

Advanced RPM With Telehealth
Integrated advanced RPM with telehealth and chatbot capabilities to improve chronic care and real-time tracking of patients.
of patients reported a better overall experience


Suicide Risk Assessment and Prevention Software
Developed RPA-powered diagnostic tool to prevent suicide risks in veterans and foster clinical decision-making.
improvement in diagnostic accuracy
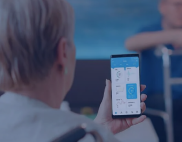

Senior Home Care Management Solution
Developed a digital home care solution that improves patient-provider communication, remote care and care coordination.
greater accuracy in health assessment